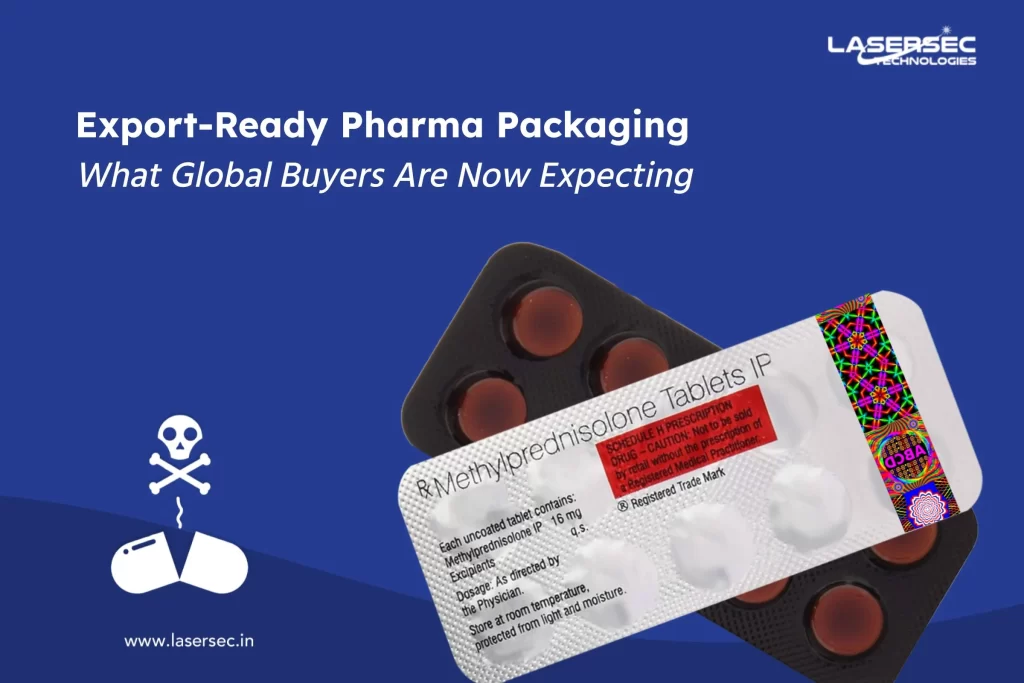
As a pharmaceutical manufacturer, you understand that packaging plays a vital role in ensuring product safety and brand protection. But here’s an important question:
Are your foils as sustainable as they are secure?
With sustainability no longer a buzzword but a necessity, the industry is shifting towards eco-friendly packaging solutions. Governments, regulators, and consumers are all demanding greener alternatives that reduce waste and carbon footprints. However, when it comes to security foils in pharmaceutical packaging, balancing sustainability and anti-counterfeiting measures is a challenge.
So, how can your packaging stay both secure and environmentally responsible?
What Are Sustainable Security Foils?
Sustainable security foils integrate advanced anti-counterfeiting features while using eco-conscious materials and energy-efficient production methods. These foils aim to:
- Reduce environmental impact while maintaining security standards.
- Meet evolving regulatory requirements for sustainable packaging.
- Enhance consumer trust by demonstrating a commitment to sustainability.
How Sustainable Foils Enhance Security Printing
- With Recyclable & Eco-Friendly Materials – These foils use responsibly sourced materials and low-impact production methods to minimize waste while ensuring durability. However, due to regulatory constraints, full recyclability is still evolving in the pharmaceutical sector.
- With Holographic Security Features – Sustainable foils incorporate high-tech holographic patterns that are extremely difficult to replicate. This enhances product authenticity and protects against counterfeiting.
- With Tamper-Evident Protection – Tamper-evident foils provide visible signs of interference, ensuring safety against theft, contamination, or fraud.
- With QR Code Authentication & Digital Verification – Foils can integrate QR codes to enable instant authentication by consumers and regulators, adding another layer of protection.
- With Track-and-Trace Compatibility – These foils can seamlessly work with track-and-trace systems to monitor pharmaceutical products throughout the supply chain. However, material compatibility with traceability technologies needs careful consideration.
Why Make the Shift?
Switching to sustainable foils offers multiple benefits:
- Regulatory Compliance: With stricter guidelines such as EU Packaging Directive, India’s EPR policies, and FDA sustainability regulations, manufacturers must adapt to avoid penalties.
- Consumer Trust & Market Appeal: Consumers prefer brands that prioritize sustainability—green packaging enhances brand loyalty.
- Cost Savings in the Long Run: Energy-efficient production reduces operational costs without compromising security.
Partner with Lasersec Technologies for Sustainable Solutions
At Lasersec Technologies, we are committed to developing innovative, sustainable security foils that protect pharmaceutical brands while supporting global sustainability efforts. Our advanced holographic foils, tamper-evident labels, and track-and-trace solutions help manufacturers achieve both security and sustainability goals.
📩 Ready to explore eco-conscious security foils?
Contact us at mktg@lasersec.in or call +91-9810213127 to learn more.