Counterfeit drugs pose a significant threat to public health and safety, requiring innovative and effective solutions to combat this global problem.
In November, the Department of Pharmaceuticals took a crucial step towards addressing this issue by publishing a gazette notice. According to this notice, starting on August 1, 2023, the 300 most popular formulation brands will be required to incorporate a barcode or quick response (QR) code on their labels.
This action, which has the cooperation of the pharmaceutical sector, aims to increase efforts in identifying and stopping the distribution of fake or counterfeit medications.
As a world-leading anti-counterfeit solutions manufacturer, we understand the urgency of addressing this issue. In this blog, we will explore the scope of the counterfeit drug problem and delve into the rise of government-mandated barcode and QR code labels as a game-changing solution.
Understanding the Scope of the Counterfeit Drug Problem:
Counterfeit drugs infiltrate the market, endangering lives and undermining public trust in the pharmaceutical industry. The World Health Organization estimates that up to 10% of medicines worldwide are counterfeit, costing the industry billions of dollars annually. These fake drugs not only fail to deliver proper treatment but can also contain harmful substances, exacerbating patients’ conditions and leading to severe consequences.
The Rise of Government-Mandated Barcode and QR Code Labels:
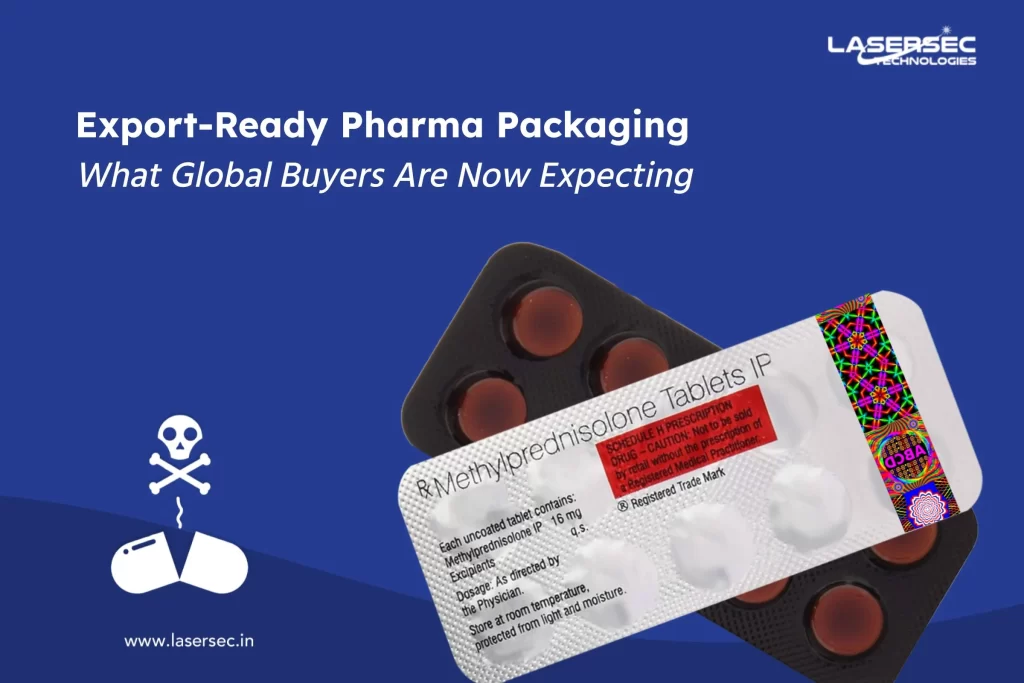
Governments worldwide have recognized the need for robust anti-counterfeit measures. They have mandated the use of barcode and QR code labels on pharmaceutical products as a means of ensuring traceability and authentication. These labels contain unique identifiers that can be scanned and verified at various stages of the supply chain, from manufacturing to distribution and retail.
Enhanced Traceability and Authentication:
Barcode and QR code labels provide an efficient way to track and trace products, enabling stakeholders to monitor their journey from production to the end consumer. This heightened visibility helps identify and eliminate counterfeit drugs from the market promptly. Additionally, through advanced authentication technologies, such as holograms and tamper-evident features, these labels offer an additional layer of security against counterfeiters.
Consumer Empowerment and Safety:
Barcode and QR code labels empower consumers by allowing them to verify the authenticity of pharmaceutical products before purchase. By scanning the labels with their smartphones, consumers can access detailed product information, including manufacturing date, batch number, and expiration date. This transparency boosts consumer confidence, ensuring that they receive genuine, safe medications.
Streamlining Regulatory Compliance:
The implementation of barcode and QR code labels streamlines regulatory compliance for pharmaceutical manufacturers and distributors. These labels enable accurate product identification, easy recall management, and compliance with serialization requirements. By automating these processes, companies can reduce errors, save costs, and efficiently meet regulatory obligations.
Future Innovations and Technological Advancements:
As the battle against counterfeit drugs intensifies, future innovations and technological advancements will further enhance anti-counterfeit solutions. Emerging technologies have the potential to revolutionize the authentication process to combat counterfeit activities more effectively.
Lasersec Technologies expertise and focus on developing innovative & world-class barcode or QR code labels and allied holographic effect. We customize holographic image with self-adhesive printed labels with other features for security & traceability. Holographic splendor is integrated with a barcode, the sequential number, printed by invisible ink, thermochromic ink etc. to make it attractive and highly secure.
These labels can be used for the packaging of products for imparting vital information like the brand owner, manufacturing details, barcodes and serial nos etc. In addition to this, with these labels you can track the goods, also helps to keep a check on the quality of the product.
We use complex holographic techniques and premium quality raw material. Because of the complexity involved during the manufacturing process, these labels cannot be duplicated. After being manufactured, a team of experienced professionals tests the entire range on various defined parameters. We offer Plain Barcode Labels, Pre-Printed Barcode Labels & also Product Barcode Labels, in different colors and designs as per the specific preferences.
Government-mandated barcode and QR code labels have emerged as a powerful weapon in the fight against counterfeit drugs.
If you’re a manufacturer or business and looking for smart packaging for your products, look no further than us. We have the knowledge, experience, and expertise to help you to find the best solution that works for your business.
Reach out to us to discuss and our team will provide you with tailored solutions and support to make the process as smooth as possible. We also One Stop Solution to fight against counterfeit and create a more secure environment for your business. With less counterfeiting, you can maintain sales and increase customer trust.
Shield your business with our advanced technology solutions
About Lasersec Technologies
We are one of the leading holographic solutions manufacturing company, that are used as an anti-counterfeiting device. Our R&D team from the production area is constantly dedicated to finding innovative solutions against counterfeits. Thus, we proudly emphasize on the fact that all our solutions are original ones without any interference from any other outside companies. We have successful business alliances located in Bangladesh, South Africa, Malaysia, Sri Lanka, Nepal, United Kingdom, and countries of Europe.
You can reach us via email at mktg@lasersec.in or by phone at +91-9810213127.