When PharmaConex 2025 opens in Cairo this September, Hall 4, Booth No. J32 won’t just be another exhibition stand—it’ll be your gateway to Egypt’s most advanced pharmaceutical packaging security and compliance solutions.
Egypt’s pharmaceutical sector is evolving fast, with growing demand for serialization, patient safety, and counterfeit prevention. Lasersec Technologies is here to show you—live—how our anti-counterfeit, track & trace, and phygital authentication solutions can protect your brand, patients, and profits.
What You’ll See Up Close
1. Next-Generation Anti-Counterfeit Solutions:
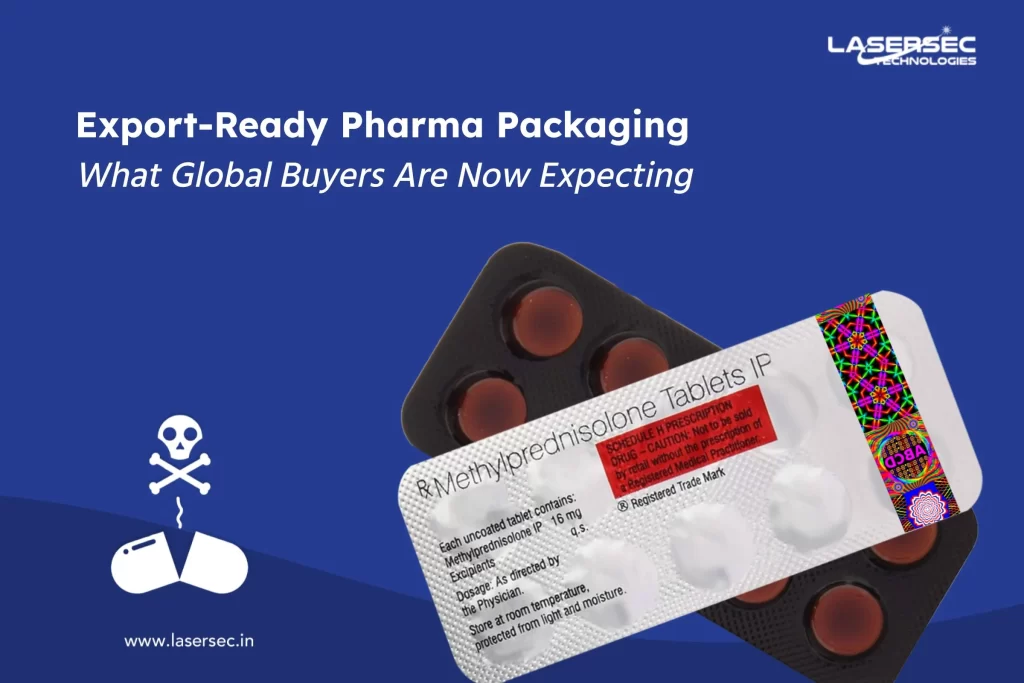
Explore our latest holographic security features designed for instant, in-field authentication. These aren’t just visual effects—they’re engineered with nano-optical structures, covert taggants, and forensic-level markers that make duplication impossible.
A recent partnership with a leading generic drug manufacturer cut counterfeit reports by 78% in just one year.
2. Track & Trace in Action:
Watch live demonstrations of serialization and secure coding integrated seamlessly into a pharmaceutical supply chain. From batch-level aggregation to unit-level GS1-compliant data capture, see how we deliver real-time product visibility, enabling end-to-end traceability from manufacturing through distribution.
Our traceability framework helped a global vaccine brand reduce supply chain discrepancies by 60%.
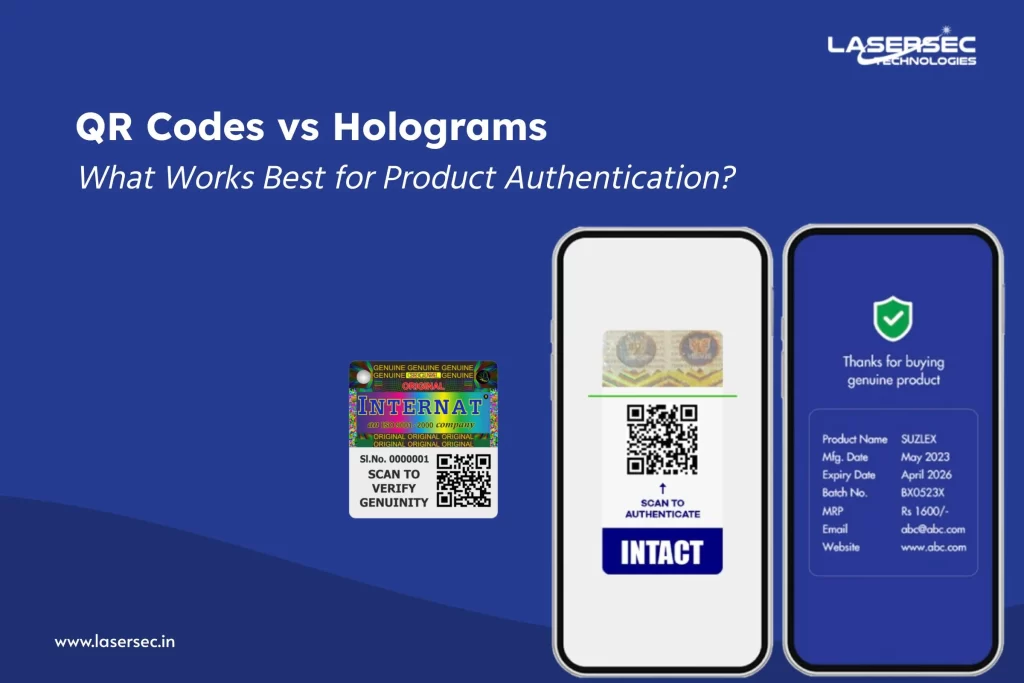
3. Phygital Authentication:
Discover how we bridge physical packaging with the digital world—allowing every stakeholder in your chain, right down to the patient, to verify authenticity via secure cloud-based checks. Our encrypted QR codes and NFC-enabled labels offer instant access to verification data, patient engagement tools, and even recall alerts—helping you improve safety and brand trust.
Why You’ll Want to Stop By
- Real Demos – Experience the technology live, not just read about it.
- Industry Insights – Stay updated on evolving serialization mandates and anti-counterfeit regulations.
- Custom Solutions – Work directly with our technical experts to solve your packaging and supply chain challenges.
This is not just about seeing products—it’s about exploring how those products can solve your real-world problems, from patient safety to market protection.
Let’s Talk at PharmaConex 2025
We believe innovation matters most when it’s practical, accessible, and tailored to your reality. Whether you want to:
- Meet global serialization requirements
- Add tamper-evident and tamper-proof packaging
- Create digital engagement channels for patients
Yes, our team will be ready to walk you through every step.
Save the Date: 7–9 September 2025
Location: Egypt International Exhibition Center, Cairo
Booth: Lasersec Technologies – Hall 4, #J32
Your brand’s reputation is priceless.
At Pharmaconex 2025, let’s make sure it stays that way.