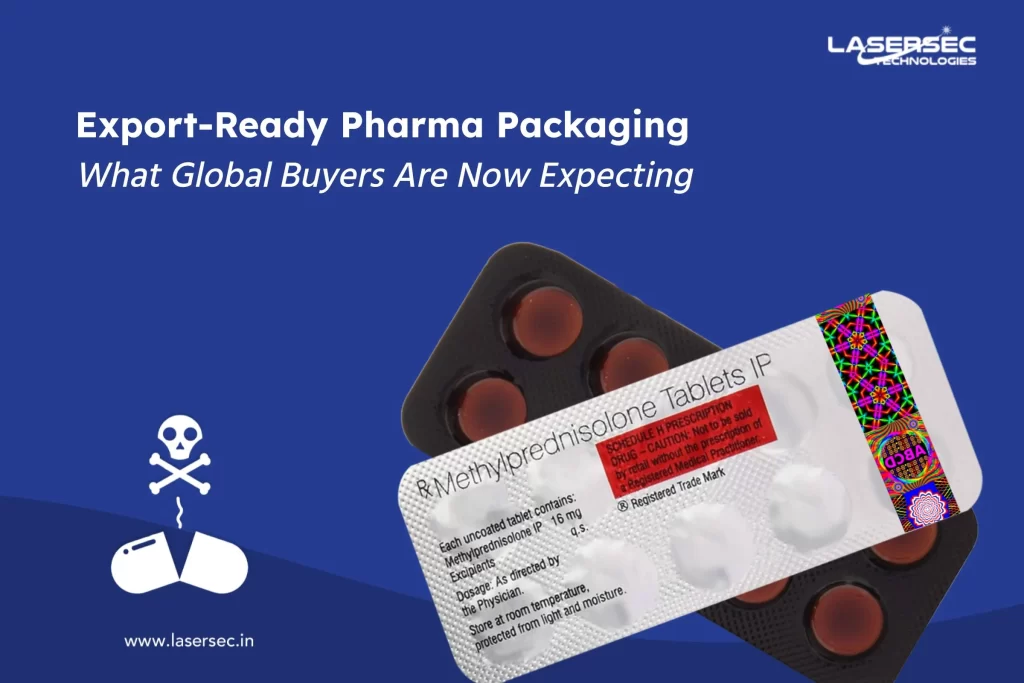
In the fast-paced world of the automotive industry, the prevalence of counterfeit auto parts is a growing concern for all of us – manufacturers, repair shops, and vehicle owners. These fake components not only hit our wallets but also pose a severe risk to the safety and performance of our vehicles. In this blog, we’re going to take a closer look at how we, as a company, deal with counterfeit auto parts. We’ll focus on how to spot them, the dangers they pose, what it means for your vehicle’s warranty, and our role as manufacturers. We’ll also discuss the importance of anti-counterfeit solutions and give you a glimpse into the future of auto parts authentication.
1. Identifying Counterfeit Auto Parts:
The initial step in protecting vehicle from counterfeit auto parts is the ability to recognize them. Counterfeit parts are often designed to look very similar to genuine ones, making visual inspection challenging.
Therefore, consumers and automotive professionals need to rely on more advanced methods, such as holographic labels, QR codes, barcodes, and other anti-counterfeit features. Seeking assistance from experts with in-depth knowledge of counterfeit auto parts is also essential for accurate identification.
2. Risks Associated with Counterfeit Auto Parts:
The risks linked to counterfeit auto parts are not to be underestimated. These components may be of lower quality and may not meet safety standards, putting vehicle performance and safety at risk. Consider the possibility of your car’s brakes failing due to counterfeit brake pads or airbags failing to deploy during a collision because of counterfeit sensors. These are not just theoretical scenarios; they have led to accidents, injuries, and even fatalities.
3. Warranty Implications:
The use of counterfeit auto parts also has consequences for vehicle’s warranty. Most vehicle warranties provided by manufacturers require the use of genuine parts and regular servicing by authorized dealerships or repair shops. The use of counterfeit parts can void warranty, leaving customer responsible for repair and maintenance costs.
Automobile Manufacturers play a pivotal role in combating the spread of counterfeit auto parts. It is their responsibility to ensure that genuine parts are readily available and to educate consumers and repair shops on how to identify these components. Leading automotive manufacturers should actively involve in developing initiatives and best practices to combat the proliferation of counterfeit parts.
In the ongoing battle against counterfeit auto parts, anti-counterfeit solutions are becoming increasingly indispensable. These solutions encompass a range of technologies and strategies designed to detect and prevent counterfeit components from infiltrating the market.
Anti-counterfeit experts, such as our company, specialize in providing cutting-edge authentication methods. By utilizing holographic labels, digital watermarks, and secure QR codes, these experts offer an effective means of verifying the authenticity of auto parts. Additionally, mobile apps can be employed to scan these codes, giving consumers instant access to vital information, such as product origins and warranties.
As technology continues to advance, the future of auto parts authentication looks promising. For instance, blockchain has the potential to create an unforgeable record of an auto part’s journey from the manufacturer to the end user. This ensures transparency and traceability, making it increasingly difficult for counterfeiters to infiltrate the market.
Identifying these fake components, understanding the associated risks, and holding manufacturers accountable for safeguarding their products are vital steps in combating this problem.
Anti-counterfeit solutions, with their advanced technologies and expertise, are a crucial part of the solution, and the future promises even more innovation in the fight against counterfeit auto parts. By staying informed and taking proactive measures, consumers and industry stakeholders can protect their vehicles and their peace of mind.
We, Lasersec Technologies, are leading anti-counterfeit solutions manufacturers that can secure the supply chains, protect the brand reputation, and ensure consumer safety.
If you’re a manufacturer or business and looking for smart packaging for your products, look no further than us. We have the knowledge, experience, and expertise to help you to find the best solution that works for your business.
Reach out to us to discuss and our team will provide you with tailored solutions and support to make the process as smooth as possible. We also One Stop Solution to fight against counterfeit and create a more secure environment for your business. With less counterfeiting, you can maintain sales and increase customer trust.
Stay proactive, stay vigilant, and invest in the right anti-counterfeit solutions to ensure a counterfeit-free future.
About Lasersec Technologies
We are one of the leading holographic solutions manufacturing company, that are used as an anti-counterfeiting device. Our R&D team from the production area is constantly dedicated to finding innovative solutions against counterfeits. Thus, we proudly emphasize on the fact that all our solutions are original ones without any interference from any other outside companies. We have successful business alliances located in Bangladesh, South Africa, Malaysia, Sri Lanka, Nepal, United Kingdom, and countries of Europe.
You can reach us via email at mktg@lasersec.in or by phone at +91-9810213127