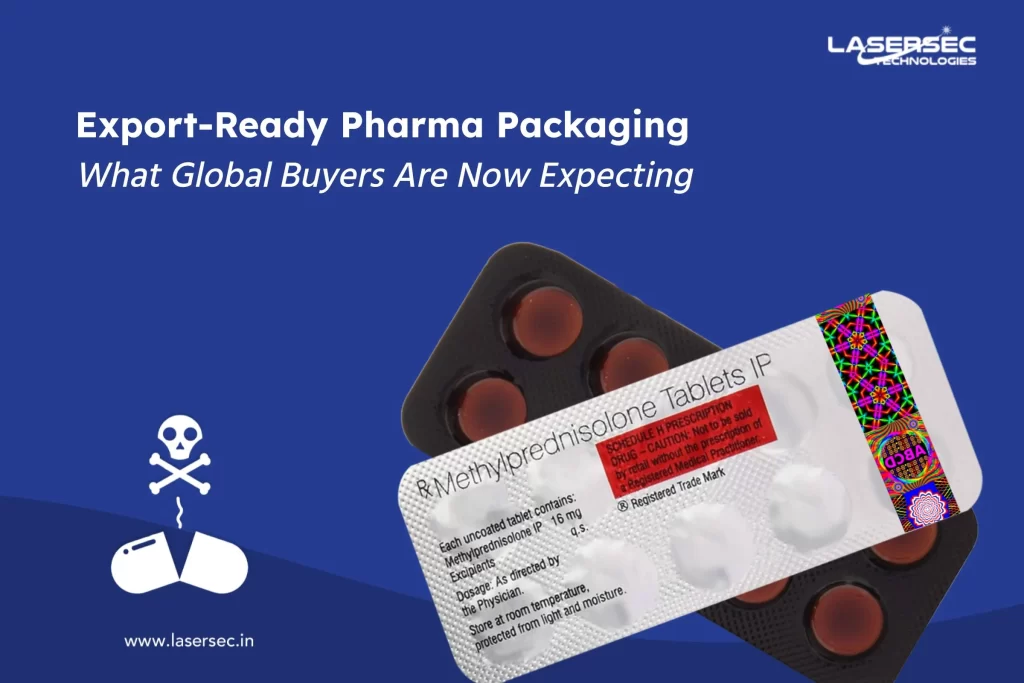
Counterfeit Drugs, Genuine Solutions!
Fake medications still remain the topmost concern in the global pharmaceutical industry. Currently, in the intricate global economy, augmentation of online transactions and complex pharmaceutical supply chains has made it even more difficult to detect such counterfeit drugs.
The pharmaceutical industry is battling simultaneously against counterfeit drugs thriving online, and also in hospitals and pharmacies. These counterfeit drugs not only affect the reputation of the drug manufacturers and cause sales loss, but they are very much dangerous to our health too.
The Imitation Spoon Is Full!
Although, the magnitude of India’s counterfeit drugs industry is still unknown, what is definite is that the situation needs immediate intervention and proper resources to stop such practices.
Presently, India has only around 1,200 drug inspectors to oversee the drug manufacturing companies that number somewhere amongst 6,000 and 15,000.
The good news is that; today, the industry is steadfast to control the problem of law enforcement; international organizations, pharmaceutical executives, and policymakers are employing a number of strong and effective strategies that are decreasing and curbing the extent of counterfeit drugs that move into users’ homes.
THE FOLLOWING ANTI-COUNTERFEIT STRATEGIES ARE QUITE PREVALENT THESE DAYS
- Holograms
Holograms can integrate three-layered safety features and become the most potent defense against faking. Holograms can offer apparent first-line validation with hidden features like micro text, UV-sensitive, scrambled images, or other dedicated inks that provide next line substantiation for trained inspectors and proper decrypting tools. - Tamper-Resistant Packing
The drug packaging is having a barrier for the entry or there is an indicator; which, if ruptured or absent, would offer audible or visible evidence to customers that tampering or meddling has occurred. For example, shrinkable bands and seals, breakable caps, film wrappers, blister packs, tape seals, etc. - Global Trade Item Number (GTIN)
In this method, a 14/13/12/8 numerically unique identification code is allocated by the manufacturer according to the GS1 allocation rules for products, or services, or trade items. It is created from a prefix from the name of the company allotted by GS1, a check digit, and an item reference number entitled by the company. - Serialized Global Trade Item Number (sGTIN)
This process includes creating a unique identification number to classify a specific product, by affixing a serial number to the GTIN of the item. - Pedigree
This is a kind of track and trace technology. It is an electronic file or a paper document that archives the particulars of circulation of a prescription drug from the manufacturer via wholesale dealings, till it is received by the distributor, which is generally a physician or a pharmacist. The individual receiving a pedigree together with the drug consignment must validate that every documented distribution took place and that the information related to the drug specification like manufacturing date and lot number is accurate. This system is intended to ascertain that prescription drugs cannot be side-tracked or substituted easily with counterfeit drugs. - Mass Serialization
This comprises the procedures of producing, coding, and authenticating the unique identity of individual drugs. Without this, the legitimacy and authenticity of the pedigree link only to the lot number comprising thousands of bottles. When serialization is united with track and trace method, it assists in the tracking of drugs through the supply chain and permits for planned identification of drugs for withdrawal. - Data Carriers
These are graphical structures used to communicate the drug identifiers and related information in the system and/or human decipherable format. A tag, label, or mark, applied at the production base depicts them. Computer decipherable formats comprise two dimensional (2D), linear barcodes and radio frequency identifier (RFID) tags.
Drug counterfeiting is a vital problem addressed by a number of countries. And the unfortunate truth is that counterfeiting drugs are hard to eradicate, and the popularity of the e-commerce and internet too are posing new challenges and threats to the drug manufacturers and governing authorities.
Although the figures will differ, WHO assesses that almost 10% of all drugs are counterfeit. But the good news is that the whole industry continues to work collaboratively and make prodigious progressions in restricting the problem. Profounder partnerships augmented co-operation, and more sharing of the resource will assist in keeping the patients safe and the fakers behind the bars.
The government has become more and more proactive in stopping counterfeiting. The Indian drug controller general has recommended a modification of the Drugs and Cosmetics Act requiring that every single drug manufacturer in the country should have a 2D barcode and a unique identification code and on the packaging, whereby, genuineness can be certified by text message. The state drug controllers are conducting surprise inspections to check the quality of the drug on a monthly basis.
And in such inspections, samples are withdrawn from pharmacies, government hospitals, and wholesalers and appropriate actions under the drugs and Cosmetics Act, 1940, are taken against the manufacturers. Recently, the Health Ministry has appointed 200 drug inspectors to keep a check, particularly on counterfeit medicines.
To avoid penalties, cancellation of licenses, prosecutions under drugs and Cosmetics Act, 1940, and piracy of drugs, the manufacturers should adopt and implement new technologies and new initiatives. For more information, log on to www.lasersec.in