Counterfeiting has been one of the biggest threats to businesses and can be very costly for businesses selling branded goods—especially in emerging markets such as Africa. If left unchecked, counterfeiters can lead to a decrease in sales and a loss of brand equity.
This blog will cover the factors contributing to counterfeiting in Africa, its impact on businesses, and solutions to this issue.
What Is Causing Counterfeiting in Africa?
The rise in counterfeiting in Africa has been attributed to a variety of factors. Some of these include the low levels of education and technical skill in manufacturing, low labor costs, high unemployment rates, and a lack of robust intellectual property (IP) enforcement.
Fake goods are often priced lower than branded products, giving consumers a reason to choose the counterfeits. This is especially true in emerging markets such as Africa where many people have low incomes.
Uncertainty in the legal framework and limited resources to implement intellectual property laws also contribute to the rise in counterfeiting. Counterfeiters can operate less riskily in markets where they are not strictly punished.
The Problem with Counterfeiting in Africa
One of the biggest issues with counterfeiting in Africa is that fake products are usually of poor quality. They may not work properly or last as long as the real product. This can lead to a customer coming back to the store to request a refund or complain about the poor quality.
Customers may also not trust the brand as much if they purchase a counterfeit product and the store they bought from. This can damage the brand’s reputation and cause long-term damage to sales. In fact, customers may be so turned off by the brand that they won’t purchase it again.
Counterfeiters also do not pay any taxes or contribute to the economy like real businesses do. This means that governments have less money to spend on public services and programs (such as healthcare and education). Counterfeiters also put those who make real products at risk for losing their jobs if counterfeiters out-compete them.
Why is Counterfeiting Bad for Businesses?
Counterfeiting can have a significant impact on a company’s bottom line. In fact, most businesses lose revenue and profit as a result of counterfeit goods. A report estimated that the global counterfeit trade is worth $1.85 trillion annually, which is equivalent to 2.5% of global trade.
Counterfeiters are able to sell their products at a low price, which means they can undercut the original sellers and take away market share. This can lead to a reduction in profits and sales for brands.
Solutions to Help Combat Counterfeiting in Africa
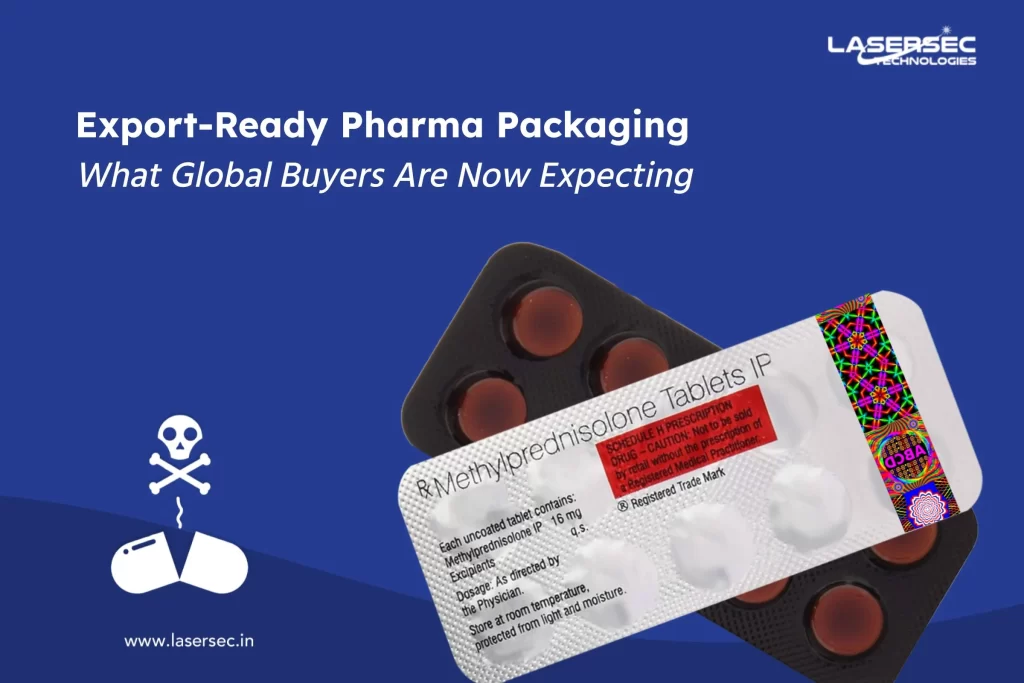
One of the biggest things that brands can do to help combat counterfeiting in Africa by using secure and safe packaging which cannot be replicated by any fake manufacturers.
Our company recently introduced a new product as “BLISTER FOIL with Security Printing” and “VOID & TAMPER PROOF LABELS,” which adds an extra layer of protection to the products and their packaging.
Another is “PHYGITAL“, a lethal combination of PHYsical and diGITAL that fights drug falsification with 100% accuracy using this innovative technology. The physical form is enhanced with the most secure yet tamper-evident labels, as well as a QR code for digital security.
About Lasersec Technologies
One of the leading and most trusted manufacturers and innovators of Anti-Counterfeiting products that provides practical solutions by constantly identifying any requirement from different aspects of the industry.
Lasersec Technologies develop and customize products based on the requirements of a specific industry. Sustained Research and Development by a qualified team of professionals supported by well-equipped facilities, and keeping abreast of the latest technology and pioneering new products and applications.
Contact us at mktg@lasersec.in or reach out on +91 –11-45637955