Counterfeits are one of the major underground markets which is growing at a fast pace. This affects people in nearly all aspects of life. Medical devices industry is also the one which has not remained untouched by its repercussions.
Counterfeits medical devices have been defined in consonance with counterfeit products as those products which are deliberately mislabelled, whereas those substandard in vitro diagnostics (IVDs) do not meet specifications and/or contain labeling errors.
These products are a nightmare to the industry as they put a patient’s life at risk without even doctors realizing it. These devices range from unsterile surgical bandages, fake surgical products made up of inferior quality components or toxic materials, faulty glucose test strips, clip cartridges, surgical mesh, personal care products, electronic components, and fake pharmaceutical formulations. The USFDA (U.S. Food & Drug Administration) has reported numerous counterfeit products in the medical devices industry over the past few years.
Do You Know?
Only 20% of countries in the world have regulation to check the development and proliferation of counterfeit medical devices industry.
To combat this growing threat, WHO has created the International Medical Products Anti-Counterfeiting Taskforce (IMPACT). This task force works to raise awareness, mobilize nations, and draft international legislation. As per WHO, more than 8% of the medical equipment in circulation are fake. The market is increasing multiple folds due to the increasing role of these devices in the healthcare industry. However, the scenario is different between developed and developing countries.
In the year 2014 in India, The Central Drugs Standards Control Organization (CDSCO) lead by central government started a reward scheme for its employees and the general public when they report fake, spurious drugs, medical devices, and cosmetics.
There is a need for medical devices manufacturers to become proactive to protect their products as counterfeits are imposing a threat to their brand equity also. It’s not only the counterfeit devices, but some authentic devices might also be fitted with faulty components causing malfunction.
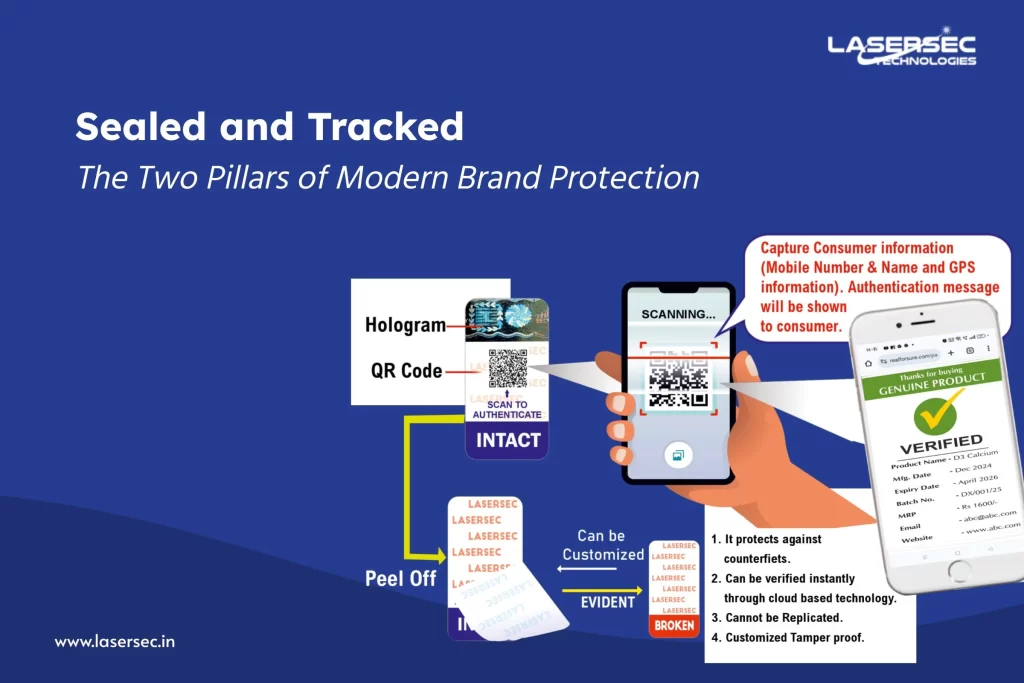
There is a need for Brand Protection Plan along with the current Good Manufacturing Practices (GMP). Protecting the credibility of a device through anti-counterfeit technology in product packaging (like Nano/microparticles, SignatureDNA, Tamper-evident opening system) is one of the ways to detect fake products.
Fight Counterfeits, Protect Yourself!!