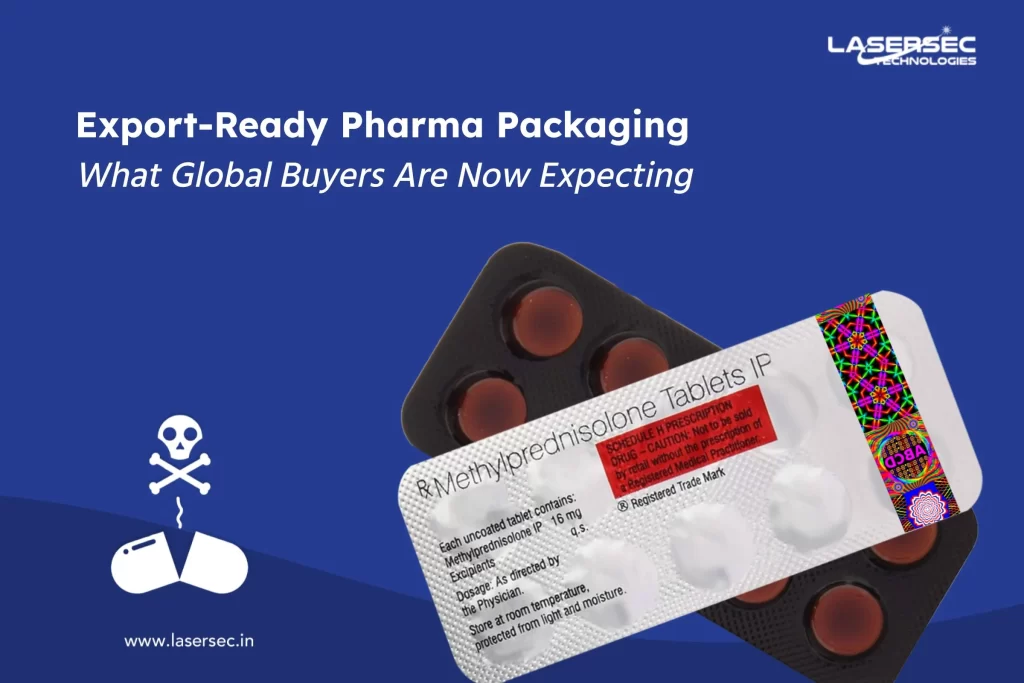
In the highly competitive pharmaceutical industry, brand protection, product integrity, and consumer trust are paramount. One of the latest innovations revolutionizing this space is holographic blister foils. These advanced packaging solutions address several challenges that pharmaceutical manufacturers face, ensuring medicines are safe, secure, and distinguishable in the market.
Tackling Counterfeiting with Advanced Security Features
Counterfeiting is a significant concern for pharmaceutical companies, leading to revenue losses and potential harm to consumers. Holographic blister foils incorporate intricate designs and optical security elements that are extremely difficult to replicate. These features not only safeguard against counterfeit products but also enhance the authenticity of the medicines, providing assurance to both manufacturers and consumers. Colour variable graphics and selective textures similar to those on currency notes add another layer of security, making counterfeiting nearly impossible.
Enhancing Brand Protection and Consumer Trust
In a market flooded with options, standing out is crucial. Holographic blister foils offer a unique visual appeal that can make a product instantly recognizable. By integrating customized holographic designs, companies can enhance their brand identity and reinforce consumer trust. These visual elements serve as a powerful deterrent to counterfeiters while fostering brand loyalty among customers. The unique aesthetics provided by these foils also offer full-proof brand authentication.
Improved Product Integrity and Tamper Evidence
Ensuring that pharmaceutical products remain unaltered from production to end-user is critical. Holographic blister foils provide robust tamper-evident solutions, making it evident if a product has been interfered with. This feature is essential for maintaining the integrity of the medication and ensuring patient safety. Additionally, these foils are manufactured in GMP-type facilities, ensuring the highest standards of quality, hygiene, and safety.
Cost-Effective and Environmentally Friendly Solutions
While security and aesthetics are crucial, cost-effectiveness and sustainability are equally important. Holographic blister foils are designed to be cost-effective, offering high security without significantly increasing packaging costs. Additionally, advancements in manufacturing processes have made these foils more environmentally friendly, aligning with the growing demand for sustainable packaging solutions.
Versatility and Compatibility with Standard Machines
Holographic blister foils are designed to be used on all standard pharmaceutical blister packing machines. They offer printing on both sides in register, with many colors including hard-to-simulate security features. This versatility ensures seamless integration into existing production lines without the need for significant modifications.
Holographic blister foils are not just a packaging solution; they are a strategic tool for pharmaceutical manufacturers to overcome challenges related to counterfeiting, brand protection, and product integrity. By adopting these advanced packaging solutions, companies can ensure their products are secure, authentic, and appealing, thereby driving consumer trust and loyalty.
If you’re a decision-maker in the pharmaceutical industry looking to enhance your packaging security, our serialization and track & trace, tamper-evident packaging, QR codes and barcodes, and holograms and security printing solutions can help you achieve regulatory compliance and protect your brand from counterfeit threats.
Stay ahead of counterfeiters and ensure the safety of your products with our advanced anti-counterfeit packaging solutions. Contact us today to learn more. You can reach us via email at mktg@lasersec.in or by phone at +91-9810213127.
Stay proactive, stay vigilant, and invest in the right anti-counterfeit solutions to ensure a counterfeit-free future.
About Lasersec Technologies
We are one of the leading holographic solutions manufacturing company, that are used as an anti-counterfeiting device. Our R&D team from the production area is constantly dedicated to finding innovative solutions against counterfeits. Thus, we proudly emphasize on the fact that all our solutions are original ones without any interference from any other outside companies. We have successful business alliances located in Bangladesh, South Africa, Malaysia, Sri Lanka, Nepal, United Kingdom, and countries of Europe.