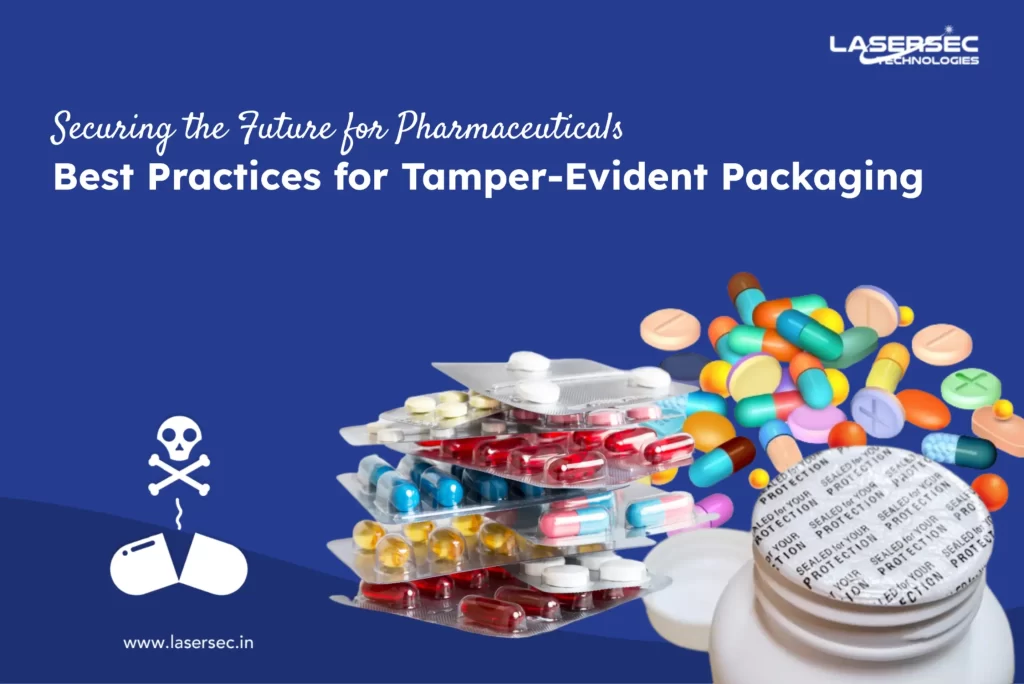
In the pharmaceutical industry, ensuring regulatory compliance with tamper-evident packaging is not just a legal requirement but also a crucial step in protecting your brand and maintaining customer trust. With increasing incidents of counterfeit products, pharmaceutical manufacturers must adopt robust anti-counterfeit solutions.
In this blog, we’ll explore the essential regulatory standards and how to meet them, the common challenges faced, and the innovative solutions available to overcome these challenges.
Overview of Regulatory Standards
Meeting regulatory standards is paramount for pharmaceutical manufacturers to ensure product safety and efficacy. Key regulatory bodies like the FDA in the United States and the European Medicines Agency (EMA) in the EU have stringent guidelines for tamper-evident packaging.
- FDA Requirements: According to the FDA, tamper-evident packaging is essential for over-the-counter (OTC) human drug products. The packaging must have one or more indicators or barriers to entry which, if breached or missing, can provide visible evidence to consumers that tampering has occurred.
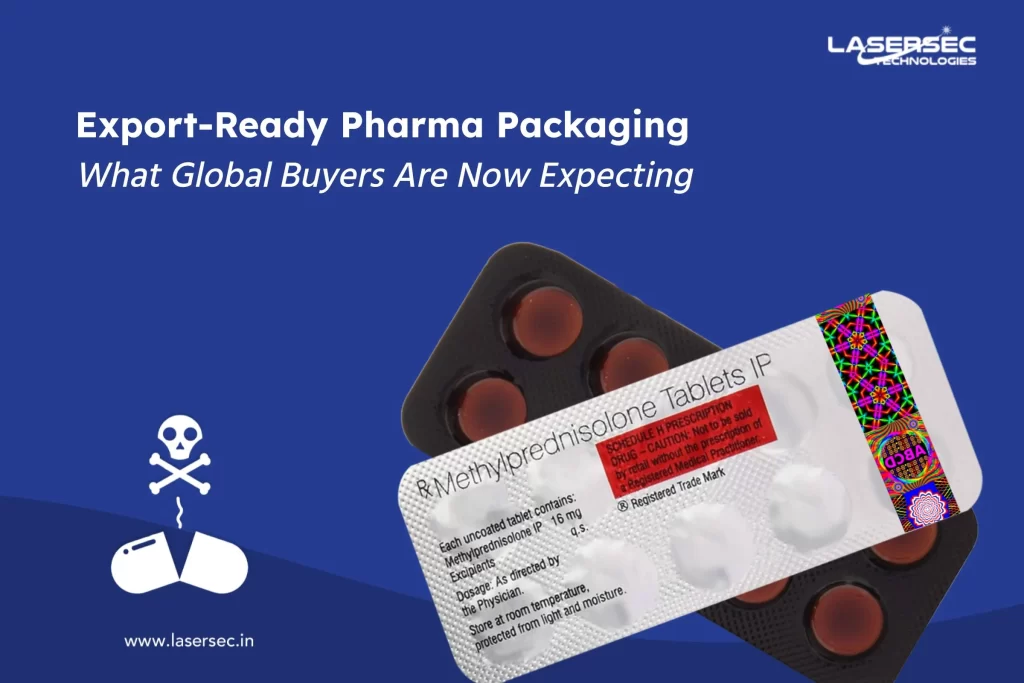
- EU Regulations: The Falsified Medicines Directive (FMD) in the EU mandates that prescription medicines should include safety features like a unique identifier (serialization) and an anti-tampering device. This helps in verifying the authenticity of medicines and ensuring that the product is not tampered with.
Common Challenges in Tamper-Evident Packaging
Pharma manufacturers face several challenges when it comes to implementing effective tamper-evident packaging:
Counterfeit Products:
The rise in counterfeit pharmaceuticals poses a significant threat to patient safety and brand reputation. Counterfeit drugs can lead to severe health risks and loss of trust in the brand.
Regulatory Compliance:
Keeping up with ever-evolving regulatory standards can be daunting. Non-compliance can lead to hefty fines, product recalls, and legal issues.
Technological Integration:
Implementing advanced packaging solutions like serialization and track & trace requires significant investment in technology and training.
Consumer Awareness:
Ensuring that consumers understand and recognize tamper-evident features is essential for the effectiveness of these measures.
Innovative Solutions to Overcome Challenges
1. Serialization and Track & Trace:
By assigning a unique serial number to each product unit, manufacturers can ensure traceability throughout the supply chain. This not only helps in complying with regulatory standards but also in identifying and combating counterfeit products.
2. Tamper-Evident Packaging:
Incorporating tamper-evident features such as seals, shrink bands, and breakable caps can provide immediate visual evidence of tampering, thereby enhancing product security.
3. QR Codes and Barcodes:
These can be scanned by consumers and supply chain partners to verify the authenticity of the product. QR codes and barcodes can also provide additional information about the product’s origin and journey through the supply chain.
4. Holograms and Security Printing:
These advanced technologies offer a higher level of security by incorporating intricate designs and features that are difficult to replicate. Holograms and security printing can be easily verified by both consumers and regulators.
Ensuring regulatory compliance with tamper-evident packaging is critical for pharmaceutical manufacturers to protect their brand and safeguard consumer health. By understanding the regulatory standards, addressing common challenges, and adopting innovative anti-counterfeit solutions, manufacturers can stay ahead in the fight against counterfeit products.
If you’re a decision-maker in the pharmaceutical industry looking to enhance your packaging security, our serialization and track & trace, tamper-evident packaging, QR codes and barcodes, and holograms and security printing solutions can help you achieve regulatory compliance and protect your brand from counterfeit threats.
Stay ahead of counterfeiters and ensure the safety of your products with our advanced anti-counterfeit packaging solutions. Contact us today to learn more. You can reach us via email at mktg@lasersec.in or by phone at +91-9810213127.
Stay proactive, stay vigilant, and invest in the right anti-counterfeit solutions to ensure a counterfeit-free future.
About Lasersec Technologies
We are one of the leading holographic solutions manufacturing company, that are used as an anti-counterfeiting device. Our R&D team from the production area is constantly dedicated to finding innovative solutions against counterfeits. Thus, we proudly emphasize on the fact that all our solutions are original ones without any interference from any other outside companies. We have successful business alliances located in Bangladesh, South Africa, Malaysia, Sri Lanka, Nepal, United Kingdom, and countries of Europe.