The European Union’s upcoming Digital Product Passport (DPP) and serialization requirements, effective from 2026, are transforming how pharmaceuticals are packaged and traced across the supply chain. For Indian pharma exporters, this means digital-ready packaging is no longer optional — it’s essential for market access, compliance, and trust.
So, how can packaging evolve fast enough to meet these expectations?
The answer lies in QR codes and tamper-evident labels — two smart technologies that bring traceability, authenticity, and transparency directly onto the pack.
1. The New EU Challenge: Digital Transparency at Every Level
Under the EU Eco-design for Sustainable Products Regulation (ESPR), every product entering the EU — including pharmaceuticals — must carry a Digital Product Passport (DPP). This digital record links each pack to verified data such as product origin, batch, composition, and regulatory certifications.
However, achieving compliance requires:
- Unit-level serialization
- Scannable digital identifiers
- Tamper-proof packaging integrity
That’s where smart labeling comes into play.
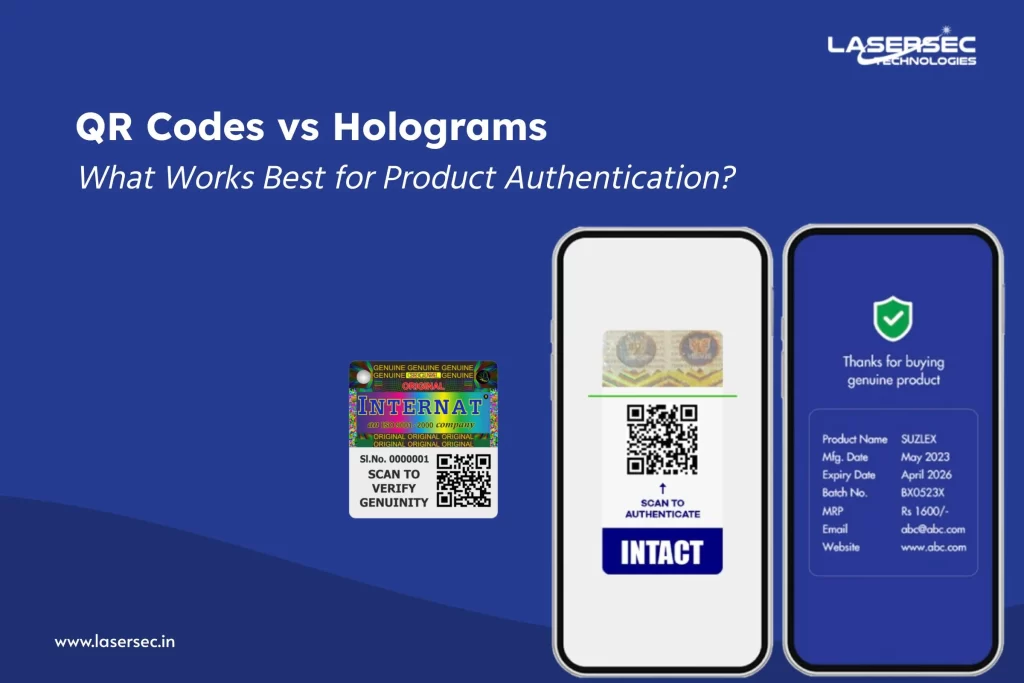
2. QR Codes: Your Gateway to Digital Product Passports
QR codes have evolved far beyond simple barcodes. Modern secure and dynamic QR codes allow real-time verification, linking every individual pack to its digital passport in the cloud.
With secure QR codes, pharma exporters can:
- Connect each product to a verified DPP record
- Enable instant verification for customs officials, distributors, and patients
- Detect counterfeits or unauthorized diversions
- Track exports throughout the EU supply chain
When integrated with serialization, these QR codes become digital fingerprints — unique, tamper-evident, and compliant with EU’s interoperability standards.
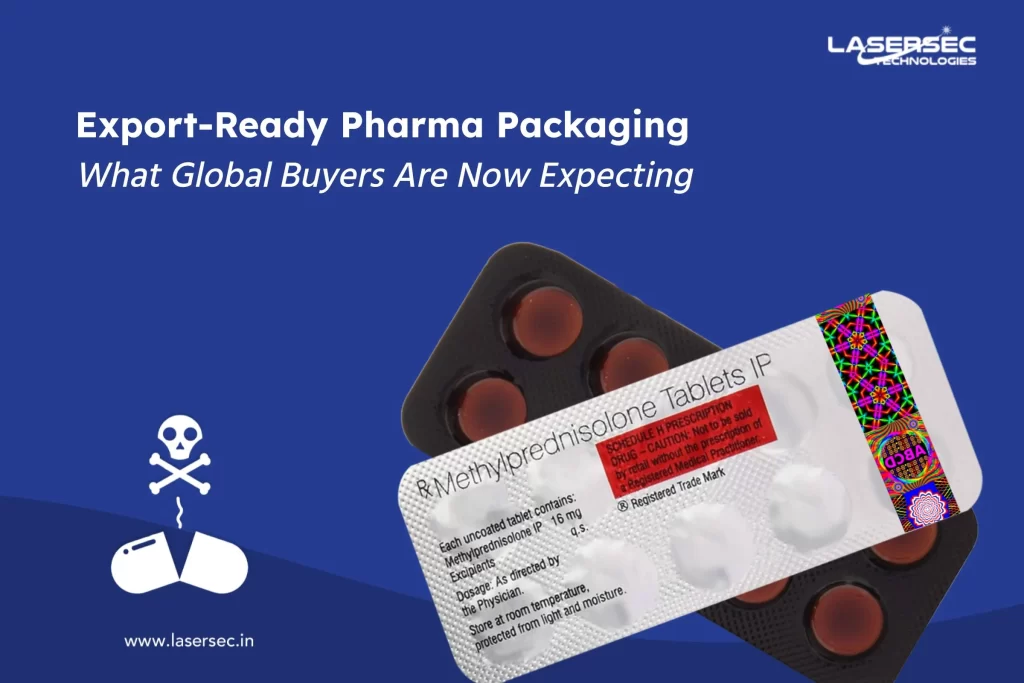
3. Tamper-Evident Labels: The Physical Layer of Trust
While QR codes protect digital identity, tamper-evident labels safeguard physical authenticity. These labels reveal visible signs — such as “VOID” marks or holographic breaks — if anyone tries to remove or alter them.
Why they’re essential for compliance:
- They ensure product integrity during transit
- Serve as proof of non-interference and genuine origin
- Complement serialization and DPP data by confirming physical security
Together, tamper-evident and QR-enabled labels bridge the physical and digital worlds, creating a robust defense against counterfeiting and regulatory breaches.
4. How These Technologies Work Together
When integrated, QR codes and tamper-evident labels deliver a two-tier protection model:
| Layer | Function | Benefit |
| Digital Layer (QR Code) | Links each pack to live data and DPP | Enables authentication and traceability |
| Physical Layer (Tamper-Evident Label) | Shows visible signs of tampering | Ensures product safety and compliance integrity |
This hybrid approach enables exporters to prove compliance instantly — both digitally and physically — meeting EU’s dual requirement of traceability and security.
5. Implementation Blueprint for Exporters
To align with the EU’s 2026 standards, Indian exporters should begin adopting these steps today:
- Adopt Dynamic QR Codes:
Implement secure, serialized QR codes linked to live cloud databases or blockchain. - Upgrade Labeling Infrastructure:
Ensure your packaging lines support tamper-evident label application and QR code printing at the unit level. - Integrate with Track & Trace Systems:
Synchronize label data with your ERP or MES to enable full supply chain visibility. - Partner with Trusted Solution Providers:
Choose technology partners experienced in anti-counterfeit, serialization, and pharma-grade packaging.
6. Beyond Compliance: Building Trust and Value
Compliance may be the starting point, but smart labeling delivers much more:
- Builds trust with regulators, distributors, and patients
- Enhances brand reputation through transparency
- Reduces counterfeit and recall risks
- Positions your company as a future-ready exporter
By embracing smart packaging now, Indian pharma manufacturers can turn regulatory pressure into a competitive advantage.
Secure, Smart, and Ready for 2026
The EU’s digital packaging regulations are reshaping global pharma trade — and Indian exporters who act early will lead the way. By combining secure QR codes and tamper-evident labels, you can ensure every shipment is authentic, traceable, and regulation-ready.
At Lasersec Technologies, we specialize in pharma-grade anti-counterfeit solutions — from dynamic QR codes to tamper-evident holographic labels and complete Track & Trace integration. Let’s help you make your exports compliant, secure, and globally trusted.
Future-proof your packaging — one secure label at a time.