You trust the seal. So does your customer.
But what if both can be faked?
In today’s hyper-competitive and counterfeit-prone market, trust is no longer a given—it must be earned, secured, and verifiable. One compromised product doesn’t just mean financial loss. It means broken trust, customer complaints, and reputational damage.
For quality assurance teams, brand managers, and packaging heads, the challenge is clear: protecting your product is protecting your brand.
The Dual-Layer Security Model
Forward-thinking brands are now adopting a two-pronged security approach: Tamper-Evident Packaging + Track & Trace Technology
One is a physical deterrent. The other is a digital watchdog. Together, they form a resilient shield against fraud.
1. What These Tools Actually Do
Tamper-Evident Packaging
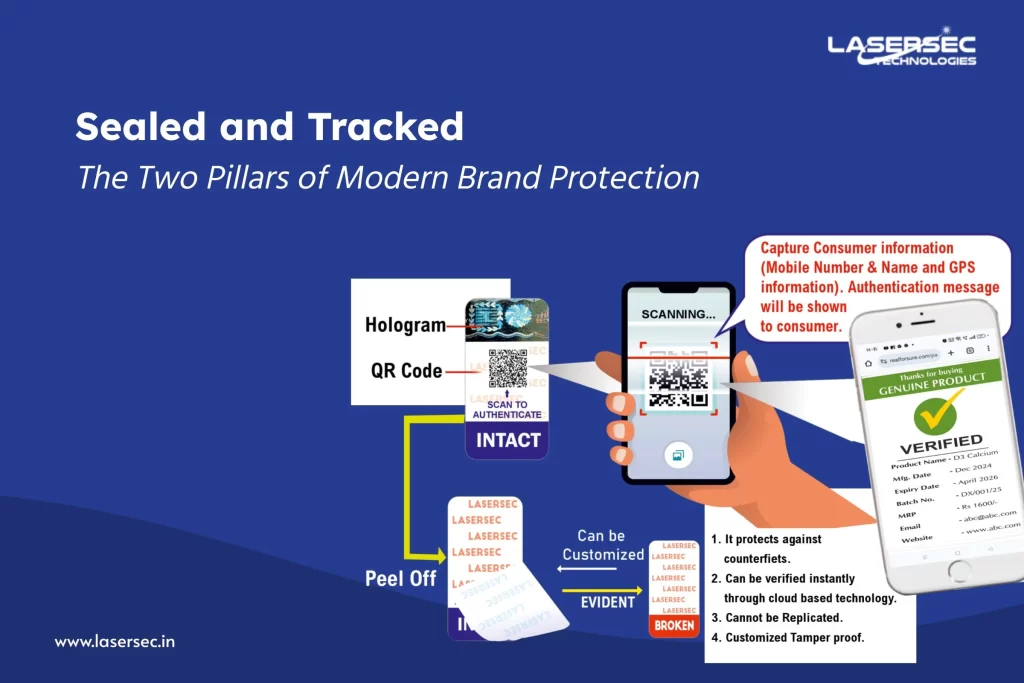
These packaging elements display clear signs of interference.
Think broken seals, VOID labels, or peeled films—immediate red flags for both consumers and supply chain teams.
2. Track & Trace Systems
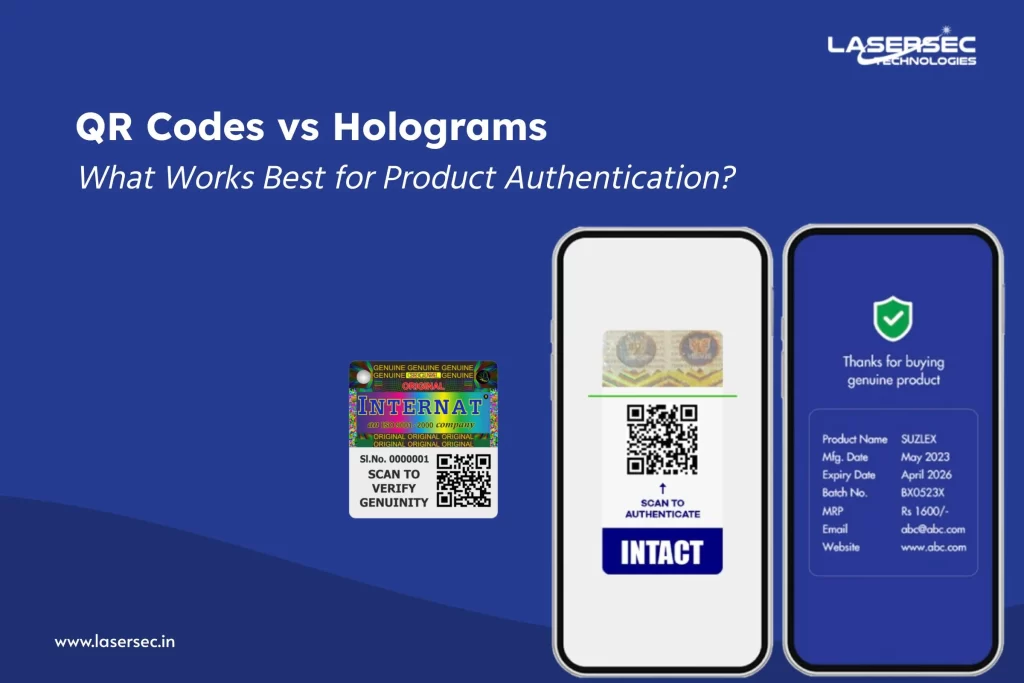
Every unit is assigned a unique serialized identity, often through GS1 barcodes or QR codes.
Each scan updates the unit’s journey from production to retail, enabling full traceability and real-time monitoring through secure platforms.
Bonus: Advanced T&T systems now integrate with cloud-based dashboards, AI-based alerts, and mobile authentication tools.
Relying on only one method is like locking your front door but leaving the windows wide open.
- Tamper seals can be replicated.
- Digital codes can be copied.
But when combined:
Physical evidence + Digital validation = A failsafe authentication system that’s far harder to breach.
Real Time Impacts
- Pharmaceuticals: A sealed pack and a scannable QR code validate medication at the pharmacy. Brands using serialization aligned with DSCSA/EU FMD have reduced counterfeit reports and met compliance faster.
- Electronics: With encrypted QR codes and tamper seals, brands can detect unauthorized resellers and trace diversion to the source. Less warranty fraud and tighter market control.
- FMCG & Personal Care: A beauty brand added tamper-evident seals and scannable codes after fake products emerged online. 38% drop in complaints, increased repurchase rates, and renewed customer trust.
Why This Approach Works
- Visible Proof:
Customers know if the product has been tampered with. - Digital Traceability:
Brands know exactly where and when a breach occurred. - Rapid Response:
Identify and contain supply chain risks in real time. - Compliance-Ready:
Stay ahead of evolving global regulations. - Brand Loyalty:
Build trust through transparency, safety, and action.
You don’t need a massive overhaul.
Start small like:
- Apply tamper-evident holographic labels to premium or high-risk SKUs.
- Add QR codes connected to a secure backend system.
- Educate your distributors and customers on scanning and verification.
Then, scale as your confidence and ROI grow.
Your Packaging Is More Than a Box
It’s your handshake, your promise, your presence in places you can’t be.
With tamper-evident packaging and track & trace working in tandem, your packaging becomes a:
- Silent Guardian
- Smart Communicator
- Powerful Brand Asset
Let’s Build Smarter, Safer Packaging – Together
At Lasersec Technologies, we specialize in merging physical security with digital intelligence. Our solutions are tailored for pharma, electronics, FMCG, automotive, and consumer brands looking to stay ahead of counterfeiting risks.