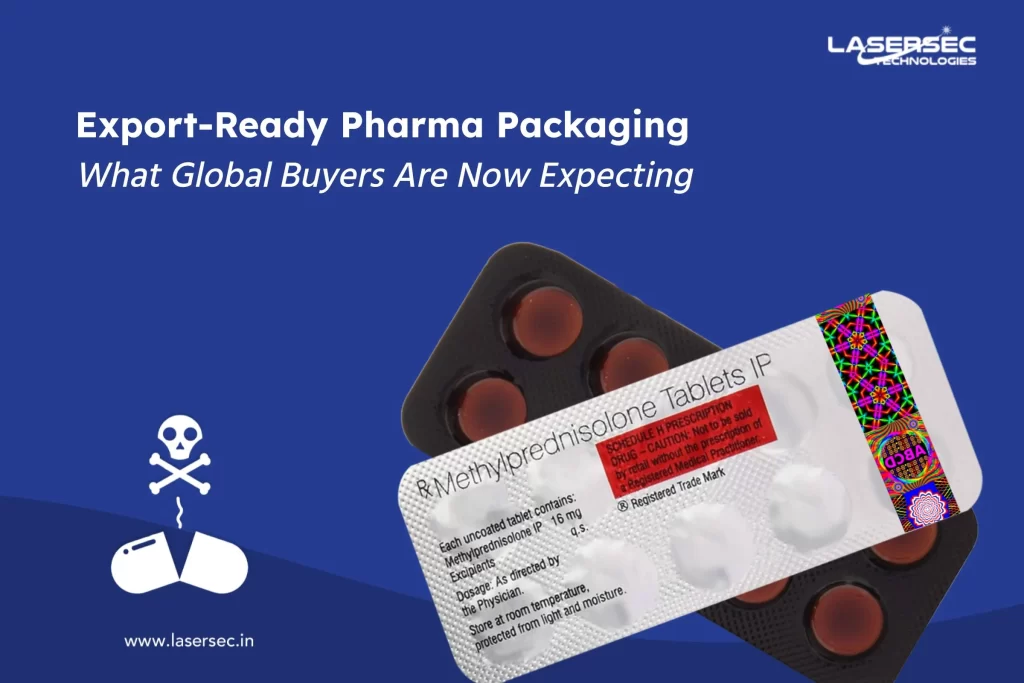
There’s no hiding away from the fact, that the rise of Counterfeit medicines in pharmaceuticals has globally impacted the industry. The adverse effects of fake medicines haven’t only left a scare on legitimate global drug suppliers but also the consumers.
But, What are Counterfeit Medicines?
Medicines that are deliberately and fraudulently mislabeled with respect to identity and/ or source. This can be applicable to both branded and generic drug-store products. Remember, Falsified medicines differ from counterfeit medicines. Falsified medicines mimic real medicines, that’s how they’re designed. But, on the other hand, medicines that do not comply with intellectual-property rights or that infringe trademark law are Counterfeit medicines
Some of the most common ways to counterfeit medicines can have –
1. Close to NIL stated active ingredients can be substituted with sometimes hazardous, adulterant, substituted ingredients.
2. Another way is, to sell under a fake or non existing brand name.
3. When legitimate drugs get expired, they are sometimes sold fresh on false dates.
What are the health consequences of Counterfeit medicines?
This is the most common doubt a person has, what happens if by any chance you consume a Counterfeit medicine. These drugs can lead to several dangerous health consequences, like side effects or unwanted allergic reactions depending from person to person and their body type. Also, due to adulteration in these drugs, it can have less level of efficacy on the body as compared to real medicine.
Wonder, how it has harmed the Pharma industry?
Counterfeit drug production and trafficking pose a significant health and safety threat to consumers. Not only that it has left a great impact on the economic growth of legitimate businesses but also they lose in terms of revenue, downtime, and replacement costs.
How counterfeiting can be stopped?
Where there’s a Problem, there’s a Solution, out there! Same for counterfeiting of Pharma medicines.
It’s not impossible for pharma manufacturers to fight counterfeit and substandard drugs on their own. It’s been made possible with the Track & Trace.
There have been many other technologies that help fight counterfeit. Some of them are – Color shifting inks, Guilloches, Blockchain Technology, Unique Identifier Marks, Anti – Alteration Devices, and many more. These systems are efficient and solve counterfeit and substandard drugs by enabling manufacturers to become more cautious of drugs throughout their supply chain cycle.
But the question arises, what about the Local Pharma distributors who either can’t afford them or aren’t aware of these technologies. That’s where Lasersec Technologies comes into the picture with their latest development of ‘Phygital’ a remarkable technology that fights against counterfeits.
Who is Lasersec Technologies?
One of the leading holographic solutions manufacturing company that is used as an anti-counterfeiting device. We provide practical solutions by constantly identifying any requirement from different aspects of the industry.
Lasersec Technologies develop and customize products based on the requirement of a specific industry. Sustained Research and Development by a qualified team of professionals supported by well-equipped facilities, and keeping abreast of the latest technology and pioneering new products and applications.
Contact us at mktg@lasersec.in or reach out on +91 –11-45637955