In the competitive pharmaceutical landscape, ensuring product integrity while maintaining robust consumer engagement is crucial for manufacturers. Tamper-proof labels not only safeguard against counterfeiting but also facilitate valuable interactions with consumers. This blog explores the multifaceted advantages of implementing tamper-proof labeling solutions, highlighting their role in compliance and market differentiation.
Understanding the Importance of Tamper-Proof Labels
Tamper-proof labels serve as a frontline defense against counterfeit products that jeopardize consumer safety and brand reputation. With the global rise in counterfeit drugs, regulatory bodies are increasingly emphasizing the importance of implementing effective anti-counterfeiting measures. By adopting tamper-proof labels, pharmaceutical manufacturers can significantly enhance product security while adhering to industry standards.
These labels are designed to show visible signs of tampering, ensuring that any unauthorized access is immediately apparent. This feature not only protects the integrity of the product but also demonstrates the manufacturer’s commitment to quality and consumer safety.
Capturing Consumer Information: A Strategic Advantage
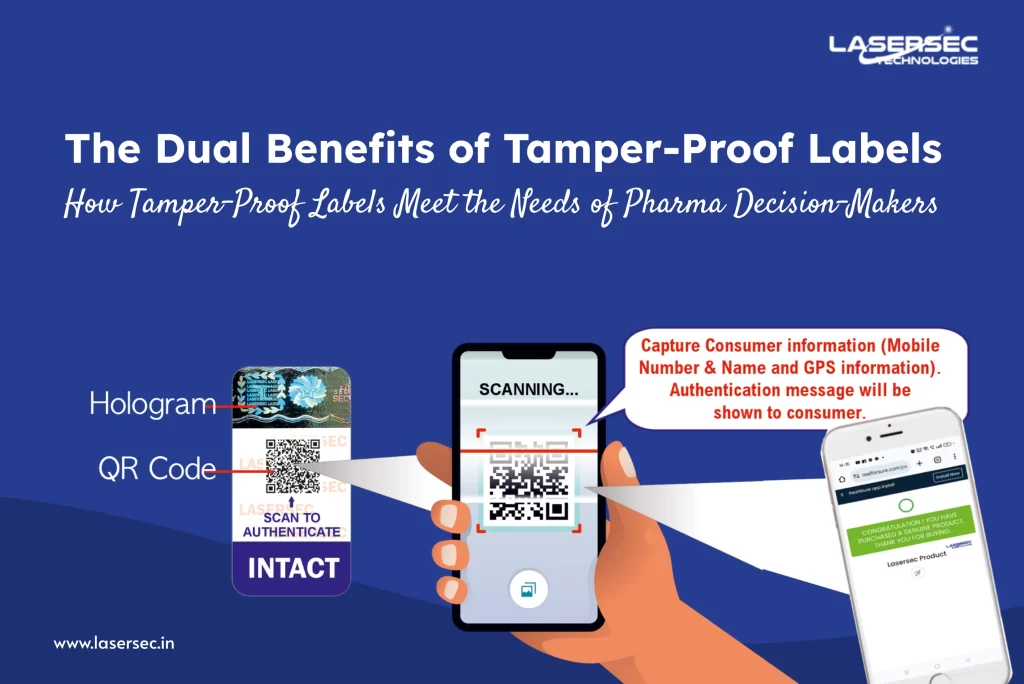
One of the innovative functionalities of modern tamper-proof labels is their ability to capture critical consumer information. By integrating data capture technology, manufacturers can gather:
- Mobile Numbers: Essential for establishing direct communication channels for marketing and feedback.
- Names: Personalizing interactions enhances customer loyalty and fosters a deeper brand connection.
- GPS Information: Understanding consumer demographics and geographic distribution enables tailored marketing strategies and more efficient product distribution.
By leveraging this data, pharmaceutical companies can refine their marketing efforts, develop targeted campaigns, and ultimately drive higher engagement levels.
The Role of Authentication Messages
When consumers scan tamper-proof labels, they receive an authentication message that reinforces the legitimacy of the product. This feature serves multiple purposes:
- Building Trust: By confirming the product’s authenticity, manufacturers can alleviate consumer concerns about counterfeit products, fostering trust and loyalty.
- Providing Information: Authentication messages can also deliver valuable insights regarding product usage, safety information, and manufacturer contact details, enhancing the overall customer experience.
This transparent communication not only benefits consumers but also strengthens the manufacturer’s reputation in the marketplace.
Key Benefits for Pharmaceutical Manufacturers
Adopting tamper-proof labels equipped with consumer data capture and authentication features presents several strategic advantages for pharmaceutical manufacturers:
- Enhanced Compliance: Implementing tamper-proof labels can help meet regulatory requirements and industry standards, ensuring compliance in an increasingly stringent environment.
- Improved Consumer Engagement: By capturing consumer data, manufacturers can develop targeted marketing strategies that resonate with their audience, leading to higher engagement rates.
- Data-Driven Decision Making: Insights gleaned from consumer interactions allow manufacturers to refine product offerings and tailor marketing strategies to meet consumer needs effectively.
- Strengthened Brand Integrity: Utilizing tamper-proof labels signals to consumers that the manufacturer prioritizes quality and safety, enhancing brand reputation and consumer loyalty.
FAQs for Pharmaceutical Manufacturers
1. What are tamper-proof labels?
Tamper-proof labels are security labels designed to indicate whether a product has been tampered with, providing visible evidence of unauthorized access.
2. How can tamper-proof labels capture consumer data?
These labels can incorporate technology that allows for the collection of consumer information, such as mobile numbers and GPS data, when scanned.
3. What benefits do authentication messages provide for manufacturers?
Authentication messages enhance consumer trust in product legitimacy, while also providing valuable information about the product.
4. Are tamper-proof labels necessary for all pharmaceutical products?
While not mandated for all products, implementing tamper-proof labels is highly recommended to protect against counterfeiting and enhance consumer confidence.
For pharmaceutical manufacturers, investing in tamper-proof labels with advanced consumer engagement features is not just about compliance—it’s a strategic move to strengthen brand integrity and foster consumer trust. By capturing valuable consumer data and providing authentication, manufacturers can differentiate themselves in a competitive market.
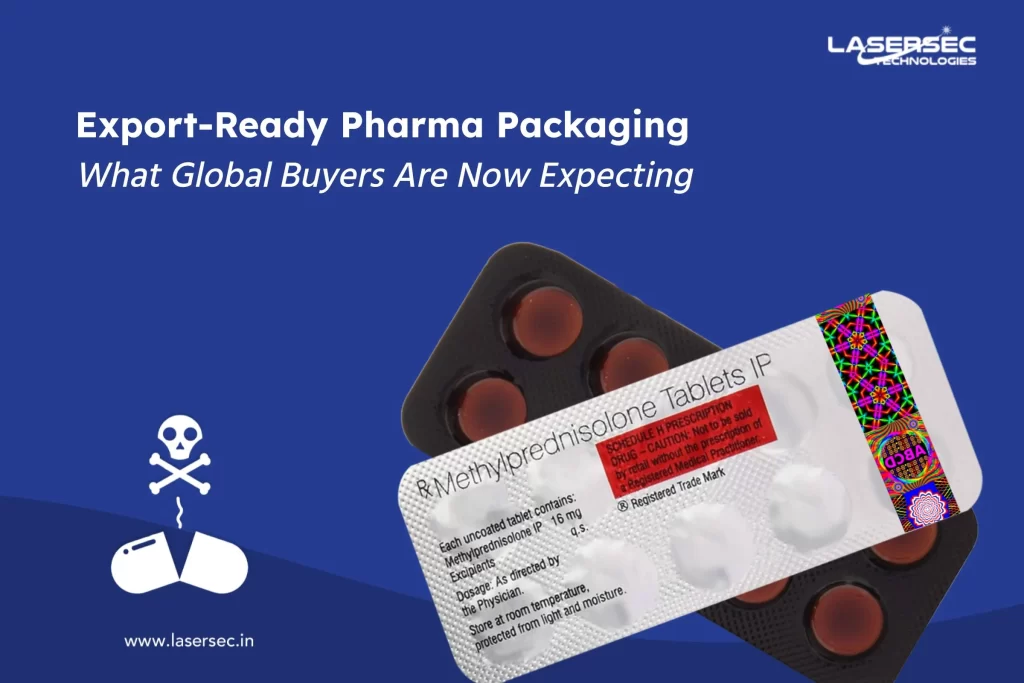
As the pharmaceutical industry battles counterfeiting and product tampering, choosing the right blister foil is crucial. By adopting advanced options like holographic designs and QR code integration, companies can enhance security measures while promoting their commitment to quality and safety.
Ready to Protect Your Brand?
For a deeper understanding of how tamper-proof labels can enhance your operational security and consumer engagement, request a demo to explore how our customized blister foil solutions can elevate your pharmaceutical packaging and protect your brand in today’s competitive market!
If you are a manufacturer or business seeking smart packaging, look no further. Our team possesses the knowledge, experience, and expertise to help you find the best tailored solution.
Reach out today, and we will provide you with customized support for a smooth process. We are your one-stop solution to combat counterfeiting and create a secure environment for your business. With reduced counterfeiting, maintain sales and boost customer trust.
Stay proactive, stay vigilant, and invest in effective anti-counterfeit solutions for a counterfeit-free future.
About Lasersec Technologies
We are a leading manufacturer of holographic solutions designed to combat counterfeiting. Our dedicated R&D team continuously seeks innovative solutions, ensuring that all our offerings are original and free from external interference. With successful business alliances in Bangladesh, South Africa, Malaysia, Sri Lanka, Nepal, the United Kingdom, and across Europe, we are committed to enhancing product security worldwide.
You can reach us via email at mktg@lasersec.in or by phone at +91-9810213127.