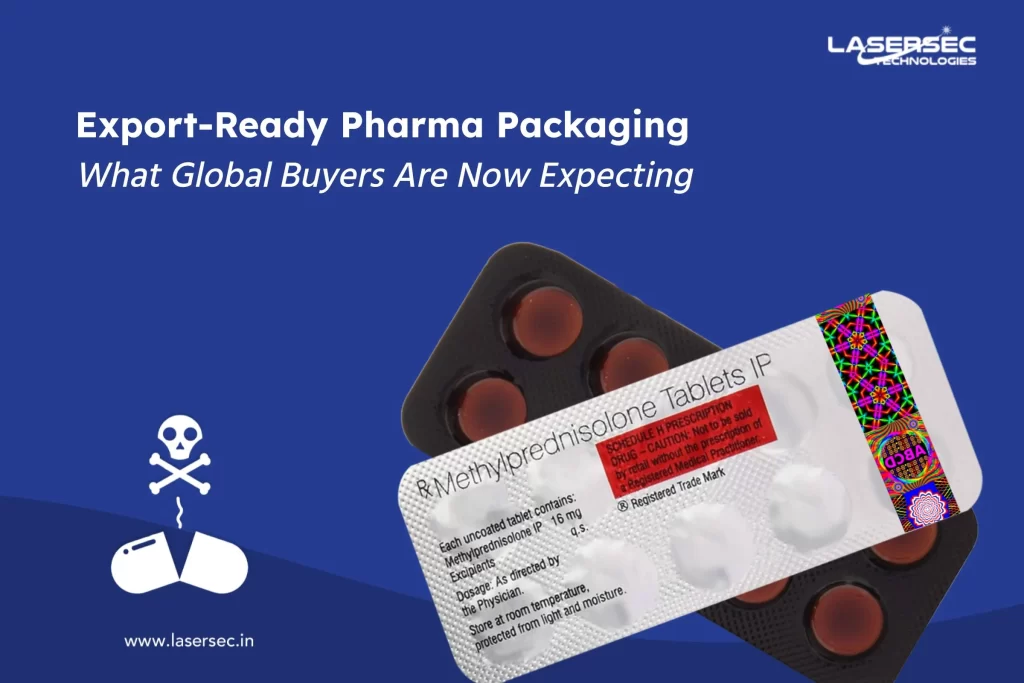
Imagine you’re driving a high-performance vehicle, confident that every component inside—from the brakes to the engine parts—is authentic, safe, and built to last. In today’s fast-evolving automotive industry, ensuring such reliability is no longer just a challenge—it’s a necessity. This is where Digital Twin Technology is transforming the way authenticity is verified in automotive parts.
Counterfeit parts have flooded global supply chains, posing serious risks to vehicle safety and performance. Faulty brakes, malfunctioning airbags, and substandard engine components don’t just lead to mechanical failures—they put lives at risk. Traditionally, manufacturers have relied on serial numbers, QR codes, and holograms to validate authenticity, but counterfeiters have found ways to replicate these too.
Enter Digital Twin Technology—a next-generation approach that bridges the gap between physical and digital verification, offering an unparalleled level of security and transparency.
Why the Automotive Industry Needs Smarter Validation Tools
Fake automotive parts infiltrate global supply chains every day, posing serious risks to vehicle performance and passenger safety. Imagine brakes that fail when needed or airbags that don’t deploy—these are the dangers counterfeit parts bring.
While traditional methods like serial numbers and QR codes have served well, they are no longer sufficient. Counterfeiters are evolving, and the automotive industry must stay ahead. Digital Twin Technology bridges the gap between the physical and digital worlds, ensuring unmatched accuracy in part validation and traceability.
What is Digital Twin Technology?
A digital twin is a virtual replica of a physical object—in this case, an automotive part. This twin is continuously updated with real-time data, capturing the part’s specifications, history, and performance. The technology leverages AI analytics, and blockchain to ensure that each component meets its original design parameters.
How Digital Twins Ensure Authenticity & Performance
- Real-Time Verification: Each part gets a unique digital identity, making counterfeiting virtually impossible.
- Supply Chain Transparency: Tracks the entire journey of the component, from manufacturer to end-user, preventing unauthorized substitutions.
- Predictive Maintenance: Monitors part performance and detects potential failures before they happen, reducing risks for drivers.
- Tamper-Proof Records: Blockchain integration ensures that all validation records are immutable and secure.
Applications of Digital Twin Technology in Automotive Parts Validation
1. Preventing Counterfeit Parts
Luxury and high-performance vehicles rely on premium-grade materials and precise engineering. By linking every component to its digital twin, manufacturers can instantly verify authenticity, reducing the risk of fake parts entering the supply chain.
2. Enhancing Supply Chain Visibility
Manufacturers and suppliers can track each part in real-time, ensuring it meets quality standards before reaching assembly lines. This eliminates risks associated with grey-market or unauthorized parts.
3. Improving Predictive Maintenance
For connected vehicles, digital twins provide real-time insights into component health. Imagine a fuel injector showing early signs of wear—digital twin data can alert the manufacturer and driver before it fails, improving vehicle longevity and safety.
Future of Digital Twin Technology in Automotive
From electric vehicle battery tracking to AI-driven diagnostics, digital twins are set to play a crucial role in the future of automotive manufacturing. Companies investing in this technology today are safeguarding their brand, enhancing supply chain integrity, and delivering safer, more reliable vehicles.
Partner with Lasersec Technologies
At Lasersec Technologies, we specialize in advanced anti-counterfeiting solutions. Our expertise in digital twins, blockchain security, and authentication technologies empowers automotive brands to eliminate counterfeit risks effectively.
Ready to explore how Digital Twin Technology can safeguard your automotive parts? Contact us at mktg@lasersec.in or call +91-9810213127 today!